Laboratory
of Atmospheric Chemistry
Lab Director
Dr.
Yannis G. Lazarou, Researcher B, Tel:
+30 210 6503623, lazarou@chem.demokritos.gr Postgraduate Students
Spyros
D. Chatziefthimiou Aristotelis
M. Zaras Collaborations
Prof. Panos Papagiannakopoulos, Department of Chemistry, University of Crete Prof. Igor I. Morozov, Russian Academy of Sciences Prof. Howard Sidebottom, University College of Dublin Prof. Claus J�rgen Nielsen, Department of Chemistry, University of Oslo Prof. Emil Ratajczak, Department of Physical Chemistry, Wrocław University of Medicine
Research Activities Experimental Determination of the Kinetics and Mechanism of Chemical Reactions in the Atmosphere
The
growing problem of environmental quality deterioration due to the release of
substances capable of inducing changes in the composition of atmosphere
constitutes one of the hottest issues worldwide. The laboratory is participating
in global efforts pointing towards the assessment of the problem by carrying out
a study of the tropospheric degradation of chemical compounds initiated by the
so-called "atmospheric detergents" (OH and Cl). The kinetics and the
mechanism of chlorine atom reactions with halogen-, sulfur- and nitrogen-
containing compounds, related with environmental problems, are studied by the
very low pressure reactor (VLPR) technique, in collaboration with the Laboratory
of Chemical Kinetics at the Department of Chemistry in the University of Crete.
Earlier studies were focused on brominated and iodinated compounds, and the
clarification of the effects of the halogen-atom adducts formation in the
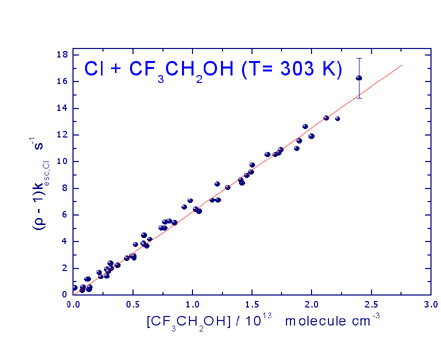
overall reaction mechanism. Recent studies involve the reaction mechanism and
kinetics of Cl atoms with fluorinated ethers and alcohols, which are proposed as
possible alternatives of chlorofluorocarbons (CFC), since the latter are very
well known to be potentially harmful to stratospheric ozone and contribute
significantly to the global warming. In addition, the reactions of hydrogen and
deuterium atoms with bromine and iodine compounds are also studied, in order to
assist in the development of a new generation of environmental friendly
fire-suppressing agents.
Development
of Theoretical Model Chemistries for the Accurate Determination of the
Thermodynamic Properties of Chemical Compounds
The availability of high performance electronic computers is continuously growing as an effective tool in achieving accurate thermodynamic properties of chemical compounds by electronic structure quantum-mechanical calculations. The knowledge of accurate thermodynamic properties permits the prediction of the molecular reactivity and gas-phase reaction mechanisms, directly applicable in the study of atmospheric degradation processes. The laboratory has extensively examined the reliability for a plethora of model chemistries employing inexpensive density-functional theory (DFT) methods and infinite basis extrapolation methods in conjunction with coupled-cluster theory - CCSD(T) -, mainly for molecules containing all four halogen atoms (F, Cl, Br, and I). The superiority of the B3P86 functional in providing very accurate bond dissociation energies was the outcome of a recent work, as well as that of the B3PW91 and B3LYP functionals is the determination of accurate enthalpies of formation. Further work aiming at reducing the cost of CCSD(T) calculations in large molecules as well as providing approximations for the scalar-relativistic and core/valence correlation effects is currently in progress.
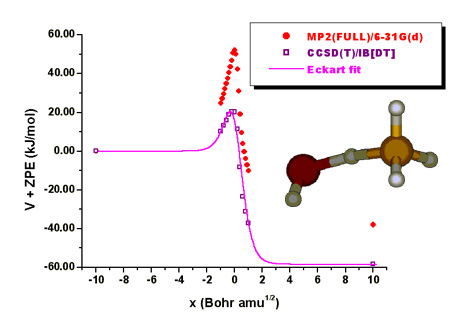
Reliable Theoretical Prediction of the Rate Constants for Gas-Phase Bimolecular Chemical Reactions
Since
the measurement of the rate constants for a wide range of temperatures is not an
easy experimental task, high-quality theoretical calculations constitute a
valuable tool. In this direction, infinite basis extrapolation methods in
conjunction with the CCSD(T) electron correlation method have been employed for
the determination of the rate constants of CH4, CH3F and
CHF3 with F, Cl and OH radicals. The calculated rates have shown a
remarkable agreement with experimental data, which is promising for the further
application of this theoretical level to practical problems related to the
atmospheric degradation of hazardous compounds, provided that sufficient
computational resources are available.
Design of Environmentally Friendly Molecules as Refrigerants and Fire-suppressing agents The
correlation of theoretically calculated molecular properties for extensive
sets of compounds with kinetic parameters of their reactions with OH radicals
and Cl atoms enables the construction of empirical expressions which may be used
to predict their tropospheric reactivity. Furthermore, these expressions may
suggest the optimal molecular structural features of molecules in order to be
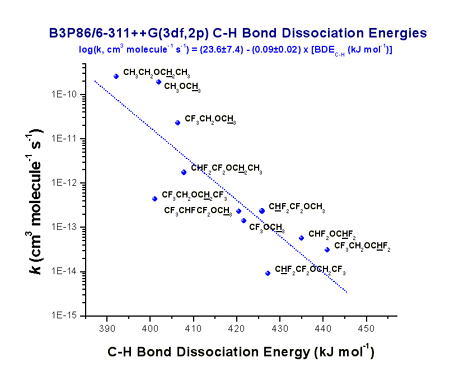
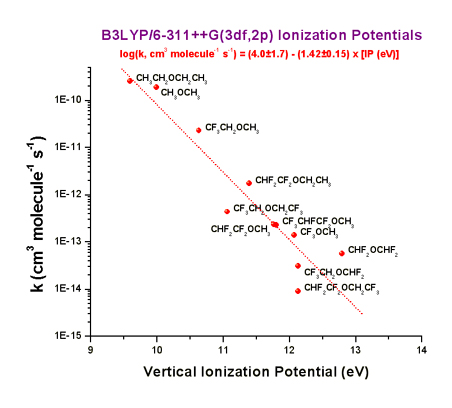
environment friendly. In a recent study, the correlation of calculated C-H
bond dissociation energies and vertical ionization potentials with experimentally determined rate
parameters for a series of fluorinated ethers was performed. The expressions
derived permit the prediction of room-temperature rate coefficients with Cl
atoms with an order of magnitude accuracy, using computationally affordable DFT
calculations. Furthermore, it was shown that for fluorinated ethers and alcohols (proposed as alternatives to
freons), high atmospheric reactivity (shortening their lifetimes and their
consequent contribution to global warming) can be achieved by the presence of
the -OCH3 or -CH2OH groups attached to a fluorinated alkyl
chain whose structure can be freely tuned to attain the optimal physical
properties.
Selected Publications
|