|
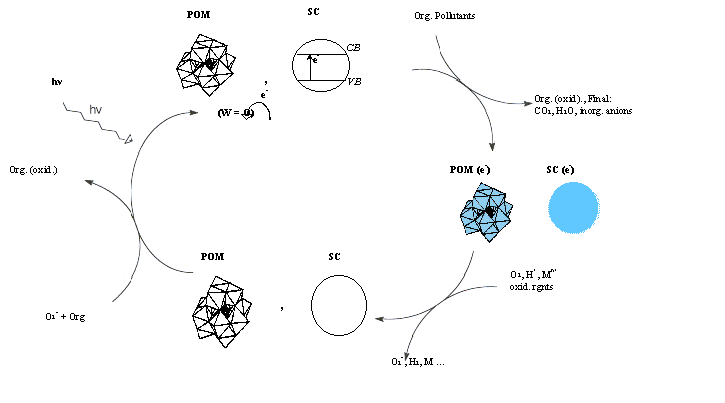
Laboratory of Catalytic Photocatalytic Processes (Solar Energy – Environment)Research Director Dr. Elias Papaconstantinou, Rank A Permanent Scientific Personnel Dr. Anastasia Hiskia, Research Rank B. Graduate Students and Research Associates Aris Troupis Stamatia Antonaraki Eleni Gkika Pigi Kormali Katerina Tsimili Research Facilities Variety of photolysis apparatus, UV-Visible-near IR spectrophotometers, Ultra Sound apparatus, Cyclic Voltametry, GC-MS, GC with various detectors (ECD, FID, TCD), HPLC with various detectors (UV, FL, Cond.), Solid Phase Extraction apparatus, Solid Phase Micro-Extraction apparatus, Ion Chromatography, Atomic Absorption, Pilot unit for decontamination of aquatic media. Research interests Catalytic photocatalytic reactions for light (solar) energy utilization, environmental detoxification (Advanced Oxidation Processes) and environmentally friendly methods. Metal oxides, mainly TiO2, and polyoxometallates (POM) mainly of W, have been used in thermal and mainly photochemical reactions according to the following photocatalytic cycle.
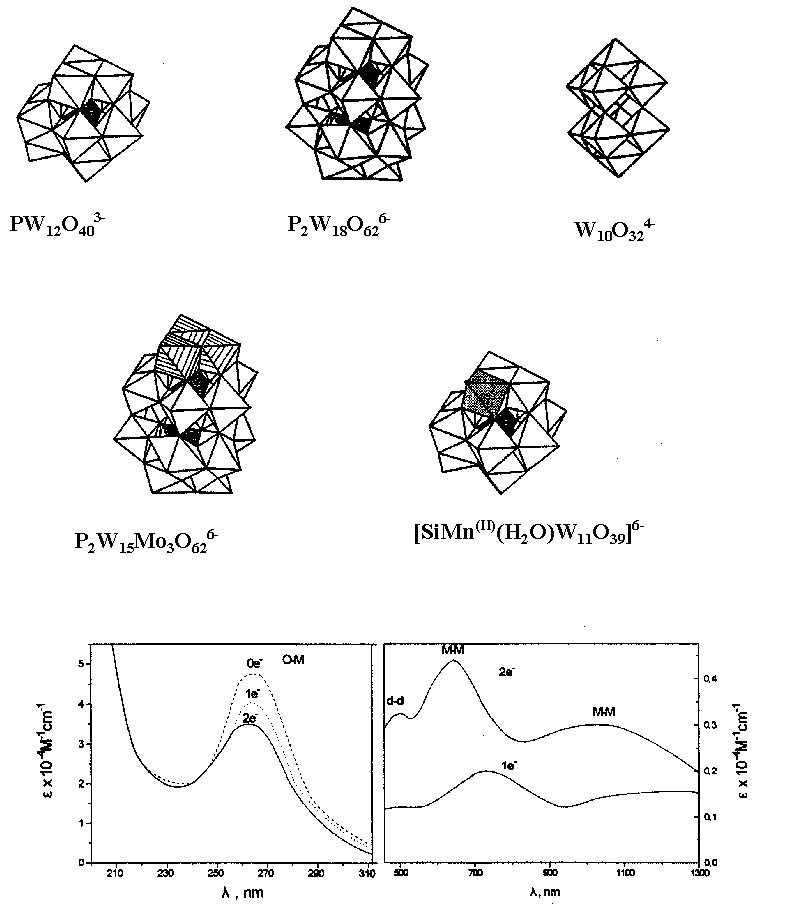
Our pioneer contribution in this photocatalytic cycle has been with the use of POM whose representative structures and spectra are shown below:
The structures of several categories of POM. PW12O403-, Keggin structure; P2W18O626-, Dawson-Wells structure. The stucture of isopoly W10O324-. Mixed Dawson-Wells structure, for instance P2W15Mo3O626- in which three W atoms have been replaced by Mo atoms. Transition metal substituted polyoxometalate (TMSP) with Keggin structure, for instance, [PW11O39Mn(H2O)]6-. Oxidized and reduced (by one and two electrons) spectra of PW12O403, showing the O® M CT band, d-d transitions and the intervalence electron transfer band (M—M CT). POM upon illumination at the O® M CT band (i.e., in UV and near-visible area) become powerful oxidizing reagents able to oxidize various organic compounds. In the process POM undergo stepwise reduction accumulating electrons that can be subsequently delivered, via thermal reactions to a variety of oxidants. This way a great variety of organic compounds are oxidized and indeed mineralize to CO2, H2O and inorganic anions and several organic and inorganic compounds can be reduced via a photocatalytic process in which POM serve as electron relays. Thus, in principle, POM can serve as decontamination photocatalysts of aquatic media removing both organic pollutants and metal ions. Under special conditions controlled photocatalytic reactions have been reported. In addition the reduction precipitation of metal ions mediated by POM may lead to formation of metal nanoparticles in which POM serve as reducing reagents and stabilizers. Our main contribution has been with the use of POM in the following areas, (representative references are given in parentheses):
Photodecomposition of ortho-chlorophenol (2.0 mM), formation of CO2 and Cl- and (insert) formation and decay of some intermediates, upon photolysis of substrate in the presence of W10O324- (0.7 mM); λ > 320 nm, pH 2.5 (HClO4), T 20 0C. (f) Reduction-removal of metallic ions. Recently, a novel photocatalytic method for the selective reduction and recovery of several metal ions from their aqueous solutions based on POM has been reported By suitable choice of POM and organic substrate, several metal ions (Ag+, Cu2+, Pd2+, Au3+, Hg2+ etc.), can be reduced to a lower or zero oxidation states. This way, photocatalytic decontamination of aqueous solutions, from organic pollutants and metal ions can take place, upon illumination in the presence of POM [New Journal of Chemistry, 2001, 25, 361; Environ. Sci. Technol., 2002, 36, 5355-5362] (g) Synthesis of metal nanoparticles. The diversified redox chemistry of POM and the fact that the reactions POM + S POM(e-) + Mn+ ® POM + M0coll (S = organic substrate, Mn+ = metal ion) can be separated in time and space or take place in one pot system, makes these compounds capable of functioning as new multifunctional mediators showing both reducing and stabilizing ability in the synthesis of metal nanoparticles [Angewandte Chemie, Intern. Ed., 2002, 41, 1911 – 1914].
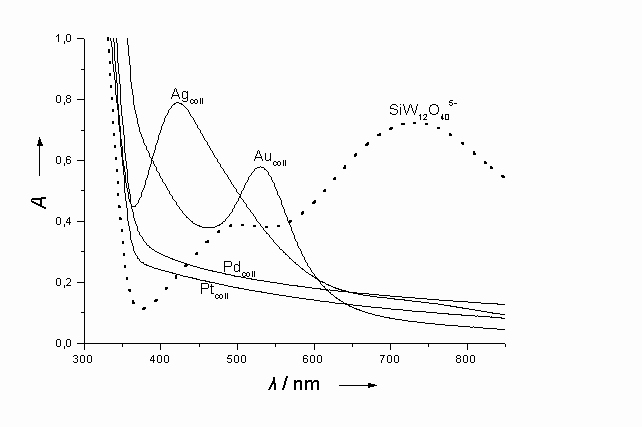
Absorption spectra of metal nanoparticles (Ag, Au, Pd, Pt) in solution, obtained after thermal reaction of photogenerated 1-equivalent reduced POM, SiW12O405-, ca 0.35 mM (spectra also shown), with the corresponding metal ions [Ag+, Pd2+, PtCl62- (0.1mM) or AuCl4- (1mM)]; pH 5.
Other research interests: (i) Immobilization of photocatalysts in optically active and/or inert substrates (ii) Sensitisation of photocatalysts towards the visible light, (iii) Use of high frequency sonar system for remediation of aquatic media [Environ. Sci. Technol., 2001, 35, 2358 – 2364]. (iii) Development of new methods of analysis for trace organic pollutants and interservices [Analyst, 1999, 124, 473; Chemosphere, 2003, 51, 69-75]
Research Grants Research programs and collaborations.
|